|
|
|
|
|
|
|
|
Photo Gallery for Anaxyrus fowleri - Fowler's Toad
| 38 photos are available. Only the most recent 30 are shown.
|
 | Recorded by: Stephanie Willis
Guilford Co. |  | Recorded by: Stephanie Willis
Guilford Co. |
 | Recorded by: C. Blackwell
Durham Co. |  | Recorded by: B. Leon-Rossi
Rockingham Co. |
 | Recorded by: Owen McConnell
Durham Co. |  | Recorded by: Sandra
Pender Co. |
 | Recorded by: Sandra
Pender Co. |  | Recorded by: J. Reynolds
Rockingham Co. |
 | Recorded by: J. Mickey
Surry Co. |  | Recorded by: J. Mickey
Surry Co. |
 | Recorded by: Matt Perry
Yadkin Co. |  | Recorded by: Matt Perry
Yadkin Co. |
 | Recorded by: Erin Dailey
Durham Co. |  | Recorded by: Simpson Eason
Granville Co. |
 | Recorded by: Steve Hall, Pat Coin, David George, and Mark Basinger
Chatham Co. | 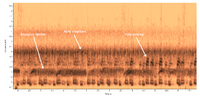 | Recorded by: Steve Hall, Pat Coin, David George, and Mark Basinger
Chatham Co. |
 | Recorded by: Stephanie Willis
Guilford Co. |  | Recorded by: Stephanie Willis
Guilford Co. |
 | Recorded by: Stephanie Willis
Guilford Co. |  | Recorded by: Salman Abdulali
Pitt Co. |
 | Recorded by: David George
Chatham Co. |  | Recorded by: Stephanie Willis
Guilford Co. |
 | Recorded by: Stephanie Willis
Guilford Co. |  | Recorded by: Stephanie Willis
Guilford Co. |
 | Recorded by: Abaigh Robinson
Orange Co. |  | Recorded by: James kelley
Wayne Co. |
 | Recorded by: James kelley
Wayne Co. |  | Recorded by: James kelley
Wayne Co. |
 | Recorded by: Savannah Hall, Dee Stuckey, and Steve Hall
Orange Co. |  | Recorded by: J. Mickey
Stokes Co. |
|

 »
»



 »
»

