Taxonomy
Class: AmphibiaOrder: CaudataFamily: PlethodontidaeSubfamily: Plethodontinae
Taxonomic Comments: The Spotted Dusky Salamander (Desmognathus conanti ) and Northern Dusky Salamander (D. fuscus ) as traditionally recognized by herpetologists have a long and complex taxonomic history, with the former originally described as a subspecies of D. fuscus . It was treated as such until genetic studies revealed that it is not closely related to the latter (Beamer and Lamb 2008; Kozak et al. 2005). According to the latest systematic treatment of these species by Pyron and Beamer (2023a) At least 10 other currently recognized species of Desmognathus were either described from populations previously considered to be D. fuscus , described as or later considered to be subspecies of D. fuscus , or later considered to be synonyms of D. fuscus before ultimately being recognized as distinct species. The latest molecular analyses have also revealed extensive cryptic diversity within both D. conanti and D. fuscus as currently conceived, with these two species constituting a polyphyletic assemblages of 13 distinct mitochondrial lineages and perhaps as many as 11 candidate species. Pyron and Beamer (2023a) provide a very comprehensive review of the taxonomic history of this group. Desmognathus species and lineages. Gene exchange has occurred between both phylogenetically sister and geographically adjacent populations, as well as between distantly related and spatially separated groups. In summary, both D. conanti and D. fuscus are now each known to represent polyphyletic assemblages of multiple, geographically well-defined candidate species that are phylogenetically interdigitated with other recognized species. The candidate species include a “mountain dusky”phenotype with smaller, gracile bodies and round tails, a lowland “dusky” type with more robust, larger bodies and keeled tails, and a smaller lowland “dusky” form often associated with ravine streams and adjacent swamps, with slenderer bodies and less heavily-keeled tails.D. conanti and D. fuscus, the authors recognized six additional species based on genetic, geographic, and morphological evidence. They also redefined D. conanti and D. fuscus . The new species are D. anicetus , D. bairdi , D. campi , D. catahoula , D. lycos and D. tilleyi . Among the newly described species, all but D. catahoula occur in North Carolina. Pyron and Beamer (2023a) reported that D. fuscus consists of three genetic lineages, two of which occur in North Carolina, and could potentially be further divided into additional species in the future. Species Comments:
Identification
Description: The Northern Dusky Salamander is a medium-sized Desmognathus that is rather nondescript. The basal third of the tail is laterally compressed and the apical half is keeled and triagonal in cross-section. There are 14 costal grooves, and the toe tips lack cornifications. Adults in many areas of the range have a relatively uniform, light dorsal stripe with a wavy or scalloped darker edge that extends onto the anterior portion of the tail. The dorsal stripe often has small, dark markings and is commonly dull yellowish brown, olive brown, or dark brown in the adults. The top of the tail is often reddish, particularly on the basal third or half. Old adults are often melanistic and are dark above with little spotting. The side of the body is lightly mottled and dusky. The venter is cream-colored overall and the melanophores are clumped to produce a mottled or salt-and-peppered pattern that becomes more conspicuous as individuals age. Adults range from 6-14 cm TL, and the maximum size of males exceeds that of females. Adult males have mental glands, enlarged premaxillary teeth, and papillose cloacal lips. The cloacal lips of the females have smooth folds. D. fuscus is restricted to our northwestern counties and mostly to the Blue Ridge and Blue Ridge Escarpment. It can be confused with other Desmognathus species that it shares stream and streamside habitats with, but note the partially keeled and triangular tail that eliminates our terrestrial montane species such as D. orestes and D. organi (both have rounded tails), and the absence of black, cornified toe-tips which eliminates semiaquatic and aquatic species such as D. Kanawha , D. monticola , D. mavrokoilius which have dark toe-tips. Pyron and Beamer (2023a) noted that it can also generally be distinguished from D. lycos based on color pattern, with adults typically possessing a wavier or more fragmented dorsal stripe (versus a straighter line in D. lycos ), less obvious differentiation between the dorsal and lateral coloration (versus a clear separation and two-toned appearance in D. lycos ), a more drab appearance in larger adults (versus frequently bold dark or orangish-brown washes on the dorsal surface of the body and tail), and a more granular or speckled ventral color pattern of yellowish, whitish, and bluish flecks (versus paler white with scattered and small dark speckles invading from the lateral margins inD. lycos ). Online Photos: Google iNaturalist AmphibiaWeb Account
Distribution in North Carolina
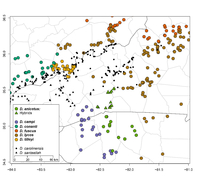
Distribution Comments: Desmognathus fuscus is a wide-ranging species that occurs in southern Canada (southeastern Ontario; southern Quebec and New Brunswick), and in the U.S. from northern Maine and other New England states southwestward to Virginia, northwestern North Carolina and eastern Tennessee. The range extends westward to western Kentucky, southeastern Indiana, southern Ohio, Pennsylvania and south-central New York. Populations are also known from southeastern Illinois and eastern Michigan that appears to be introduced (Pyron and Beamer, 2023a). Populations in North Carolina are found in the northern mountains and foothills where they are parapatric with D. lycos to the east (see distribution map above and the map on the D. conanti account). Distribution Reference: (Pyron and Beamer, 2023a).County Map: Clicking on a county returns the records for the species in that county.
GBIF Global Distribution
Key Habitat Requirements
Habitat: The Northern Dusky Salamander is semi-aquatic and lives in springs, seeps, and streams in both wooded ravines and bottomland forests (Petranka 1998). The eggs are laid in or near seepages and small streams, and the larvae are aquatic. Rocky streams in forested, protected watersheds with minimal siltation provide optimal habitats for this species. Biotic Relationships: Desmognathus fuscus lacks chemical defenses against predators and is likely consumed by many predators such as crayfishes, raccoons, semiaquatic snakes, and owls. Uhler et al. (1939) found that dusky salamanders are occasionally taken by the Common Watersnake (Nerodia sipedon ) and Common Gartersnake (Thamnophis sirtalis ). Individuals often remain immobile when uncovered, which is an antipredator behavior that makes them less conspicuous. They can also rapidly flee and quickly disappear beneath cover. Individuals will often attempt to bite attacking predators such as small snakes, and may autotomize the tail if it is grabbed by a snake or other predator (Whiteman and Wissinger 1991).
Life History and Autecology
Breeding and Courtship: The adults mate during both the autumn and spring in Virginia and New York (Bishop 1941, Organ 1961a) and females breed every year in many populations (Danstedt 1975, Spight 1967). Females in mountainous regions of southwestern Virginia appear to have biennial reproductive cycles and the sex ratio is about 1:1 (Organ 1961a). The adults engage in a set of rather stereotypic courtship behaviors that appear to be essentially identical for D. conanti , D. santeetlah , and D. fuscus . The following aspects of courtship are from Petranka (1998).Reproductive Mode: The females nest in or along the edges of streams and seepages and lay their cream-colored to whitish eggs in globular or grape-like clusters. A female will often construct a small depression beneath a cover object before laying her eggs. The freshly laid eggs have ova that are 2.5-3.5 mm in diameter and are surrounded by three envelopes. The outer envelop of each egg is drawn out into a short stalk that is attached either to support structures or other eggs or stalks. Females attach their eggs to the undersides of rocks in streams, in cavities in stream banks, and under rotting logs, in leaf mats, or in clumps of moss near the stream edge (Petranka 1998). Snodgrass et al. (2007) found that females in a Maryland watershed were most likely to nest in headwater habitats with narrow stream channels. The eggs in this study were laid in stream banks 1-88 cm from the water's edge, and were often placed in areas where the stream section was relatively wide and deep. The hatchlings of females that nest in headwater sections of streams generally suffer little or no predation by fishes and are less likely to drift downstream into fish-filled habitats. Aquatic Life History: The hatchlings have large amount of yolk stores that may take over 2 months to absorb (Orr and Maple 1978), but they begin feeding on tiny prey long before absorption is complete (Montague 1979; Wilder 1913). The larvae feed on small prey such as copepods, chironomid larvae, plecopteran nymphs, collembolans, mites, and fingernail clams (Burton 1976, Wilder 1913). Larvae in four populations in northeastern Kentucky and southern Ohio grew very little during the fall and winter following hatching (Juterbock 1990). Growth rates accelerated beginning in mid to late April and continued until larvae metamorphosed in June or July after about a 9-month larval period. In general, larvae in southern populations studied by Juterbock (1990) grew faster, transformed slightly sooner, and were larger at metamorphosis than larvae farther north where conditions were cooler. Terrestrial Life History: The juveniles and adults are active on the ground surface at night during the warmer months of the year, particularly during the first hour after dark when prey are most active (Holomuzki 1980). They move into underground retreats during the winter where they occasionally take prey (Petranka 1998). Adults studied by Ashton (1975) in Kentucky moved into winter retreats that were 12-25 cm deep and occasionally gathered in dense aggregates. D. fuscus larvae in adults he uncovered in underground winter retreats in New York, and Wilder (1913) found Desmognathus larvae that were likely conspecifics in the stomachs of two adults collected in the field. Brooding females have greatly reduced foraging rates relative to nonbrooding animals (Krzysik 1980, Montague and Poinski 1978), which explains why in many populations they breed every other year.
General Ecology
Population Ecology: Local populations are generally confined to seepages, springs and small headwater and first-order streams where fish are either absent or occur at low densities. The population dynamics of these relatively isolated populations are poorly documented. Patterns of survival may vary in local populations. Males in southwestern Virginia exhibited Type I survivorship in which the probability of dying increases slightly with age (Organ 1961a). Sexually mature females have lower survivorship than males, which may reflect the costs of brooding. Danstedt (1975) studies six populations in Maryland and found that age at sexual maturity, reproductive rates, sex ratios, age structure, survival rates, and body sizes were very similar across populations. Survivorship was slightly lower during the first 3 years of life than in later years. Nonetheless, age-specific survivorship tends to be relatively constant throughout life, particularly for the males. Community Ecology: Organ (1961a) presented distributional data which suggest that D. fuscus may be excluded from some stream habitats in Virginia where both D. monticola and Desmognathus kanawha co-occur.
Adverse Environmental Impacts
Effects of Pollution: Like many aquatic organisms, dusky salamanders are sensitive to timbering, urbanization, stream pollution and siltation. Gore (1983) found that D. fuscus larvae were absent from many streams draining coal strip mines in eastern Kentucky and Tennessee. Stream siltation and high metal concentrations appeared to be the primary factors reducing or eliminating populations from these streams. Brady (2016) surveyed salamander communities on 45–65 year-old forests that had become established on abandoned coal surface mine sites in Ohio and compared them with unmined reference sites. He obtained similar results and found that stream-breeding species such as Eurycea longicauda , E. bislineata , and D. fuscus were well-represented in reference forests but were missing from all reforested mine sites. Persistent acid drainage from the mined sites was the presumed cause.
Status in North Carolina
NHP State Rank: [S4]Global Rank: G5Environmental Threats: This and many other stream-dwelling salamanders thrive in clean streams with well-developed deciduous forest in the adjoining floodplain and throughout the watershed. These habitats can be seriously degraded by excessive sedimentation that embeds rocks and destroys nesting habitat. Deforestation eliminates leaf litter inputs that support stream invertebrates and provide cover and nesting sites for salamanders.

 »
»

 »
»