|
|
|
|
|
|
|
|
Photo Gallery for Hyla cinerea - Green Treefrog
| 44 photos are available. Only the most recent 30 are shown.
|
 | Recorded by: B. Bockhahn
Sampson Co. |  | Recorded by: Louis Skrabec
Guilford Co. |
 | Recorded by: R. Newman
Carteret Co. |  | Recorded by: Guy McGrane
Watauga Co. |
 | Recorded by: Paul Hart, Tom Worden
Harnett Co. |  | Recorded by: Steve Hall, Pat Coin, David George, and Mark Basinger
Chatham Co. |
 | Recorded by: David George, Jeff Niznik
Chatham Co. |  | Recorded by: Judith West
Randolph Co. |
 | Recorded by: David George
Orange Co. |  | Recorded by: David George
Orange Co. |
 | Recorded by: David George
Orange Co. |  | Recorded by: Jack V.
Beaufort Co. |
 | Recorded by: R. Newman
Carteret Co. |  | Recorded by: Chuck Smith
Davidson Co. |
 | Recorded by: Mark Basinger
Brunswick Co. |  | Recorded by: David George
Durham Co. |
 | Recorded by: Travis McLain
Anson Co. |  | Recorded by: Travis McLain
Cabarrus Co. |
 | Recorded by: Travis McLain
Cabarrus Co. |  | Recorded by: Mark Basinger
Wilson Co. |
 | Recorded by: Rob Van Epps
Mecklenburg Co. |  | Recorded by: R. Newman
Carteret Co. |
 | Recorded by: Mark Shields
Duplin Co. |  | Recorded by: Carrie DeJaco
Stanly Co. |
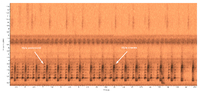 | Recorded by: Steve Hall and Jim Petranka
Richmond Co. |  | Recorded by: Steve Hall
Durham Co. |
 | Recorded by: Mark Shields
Dare Co. | 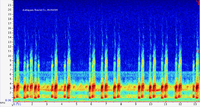 | Recorded by: Jim Petranka and Becky Elkin
Beaufort Co.
Comment: Spectrogram of a pair with alternating calls. |
 | Recorded by: Steve Hall
Durham Co. |  | Recorded by: R. Newman
Carteret Co. |
|

 »
»





























