Taxonomy
Class: AmphibiaOrder: CaudataFamily: PlethodontidaeSubfamily: Plethodontinae
Taxonomic Comments: Members of the genus Desmognathus are commonly known as dusky salamanders because of their overall dark brown or dusky ground color. Like many plethodontid salamanders, they have proven to be a taxonomically challenging group that contains several species complexes. Kozak et al. (2005) documented 35 major lineages in the eastern US, even though only 22 species were formally recognized by taxonomists in 2021. This suggests that there are numerous cryptic species that remain to be described. A more recent comprehensive molecular survey of populations in the eastern US by Beamer and Lamb (2020) indicate that at least 45 major lineages or clades are present. D. quadramaculatus ) as a species that can be identified by its relatively large size, stocky build, keeled and laterally compressed tail, cornified toe-tips, and black belly. If differs from the Shovel-nosed Salamander (D. marmoratus ) in several traits, the most obvious being differences in the shape of the internal nares. Recent molecular studies by Beamer and Lamb (2020), as well as several previous studies, have shown that these two species are not monophyletic. Instead, populations of both species are interspersed within a strongly supported clade that contains all populations of D. quadramaculatus , D. marmoratus , and a third species, D. folkertsi . This major clade contains two subclades, including one that includes all D. quadramaculatus populations sampled south of the Pigeon River, as well as all D. marmoratus populations sampled in the Apalachicola and Savannah River drainages. The second subclade includes all populations of D. quadramaculatus sampled from east of the Tuckasegee River drainage basin (with one exception) as well as all populations of D. marmoratus sampled outside the Apalachicola and Savannah River drainages. In some cases populations of D. quadramaculatus are more similar genetically to D. marmoratus than to other D. quadramaculatus populations. Collectively, the research by Beamer and Lamb (2020) and Pyron et al. (2020) indicates the presence of a species complex that involves all three currently recognized species. D. folkertsi , and four new species (D. amphileucus , D. gvnigeusgwotli , D. kanawha and D. mavrokoilius ) that replace what was traditionally known as D. quadramaculatus and certain populations of D. marmoratus . These species are best identified in the field using the site of collection (see distribution map above). Species Comments: Desmognathus amphileucus is a southern member of the Black-bellied Salamander complex and was originally described by Bishop (1941) as a subspecies of Desmognathus quadramaculatus .
Identification
Description: Desmognathus amphileucus closely resembles other members of the Black-bellied Salamander complex and is best identified by its geographic range, its size, and certain morphological features. Like other members of the complex, it is a large, stocky Desmognathus with a black belly and usually two rows of light dots along each lower side of the body. The keeled tail is laterally compressed, and the toe tips are black and cornified. The dorsal ground color is typically black or dark brown with dull rusty or chocolate-brown blotches. Pyron and Beamer (2022) noted that the large adults are distinctive in often having a venter that is solid jet-black. The venter lacks any secondary coloration, versus the salt-and-pepper, mottled, or slaty-gray venters that commonly occur in the other members of the complex. In addition, the dorsal and lateral surfaces sometimes have a solid, vibrant chocolate-brown color with little to no patterning, versus a darker and often more mottled or faintly patterned brown or black in the other species. D. amphileucus is found in the southwestern counties and is generally geographically isolated from other members of the complex. There are a few sites where it coexists with D. folkertsi in southern Macon County, and two very small contact zones with D. mavrokoilius in the northeastern portion of the range (see Pyron and Beamer, 2022). At sites where the two species are syntopic, D. amphileucus can be separated from D. folkertsi by 1) a larval forelimb length/snout-vent length ratio greater than 0.2 (versus less), 2) its larger size at metamorphosis (44–56 mm versus < 40 mm), 3) its larger size upon ventral darkening (> 60 mm versus 40 mm for D. folkertsi ), 4) its larger adult size (79–120 mm SVL versus 56–85 mm), 5) the presence of a red dorsal tail stripe in subadults (absence in D. folkertsi ), 6) occasional olive green dorsal coloration in immature specimens (absence in D. folkertsi ), 7) the absence of a discernable pattern of dorsal blotches (occasional presence in D. folkertsi ) and 8) fixed differences in several allozyme loci as well as mitochondrial and nuclear loci (Pyron and Beamer, 2022). This species also has a much larger maximum body size (up to 120 mm SVL) than the other members of the complex, and has a proportionally wider head than all species except D. folkertsi . This species are best identified in the field using the site of collection (see distribution map above). Desmognathus species that are not members of the Black-bellied Salamander complex, and have whitish gills and dark toe tips. The bellies of very young juveniles are whitish and acquire dark pigmentation within several months to a year after metamorphosis. Care needs to be taken to avoid confusing the young juveniles with light bellies with other Desmognathus species that have laterally compressed tails.Online Photos: Google iNaturalist Observation Methods: The adults are most easily collected by turning large rocks or other cover in streams. At night, they often can be observed with their heads or upper bodies protruding from burrows and cover objects. AmphibiaWeb Account
Distribution in North Carolina
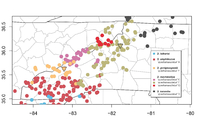
Distribution Comments: This species occurs primarily in the Blue Ridge Mountains in southwestern North Carolina, extreme northwestern South Carolina, extreme southeastern Tennessee, and northeastern Georgia (Pyron and Beamer 2022). A few isolated populations occur in the Piedmont of Georgia and South Carolina that are probably the result of bait-bucket introductions (Martof 1953; Valentine 1974), while a population in Madison Co., Georgia that is isolated to the south of other Piedmont populations appears to be of natural origin (Camp et al. 2013). D. amphileucus occurs from mostly west of the French Broad River, and just south of the Great Smoky Mountains, south and southwestward to the Tennessee, Georgia, and South Carolina borders. The known range includes all of Cherokee, Graham, Clay, Macon, Jackson, and Transylvania counties, as well as extreme southern Haywood County, southern Henderson County, western Polk County, and extreme southeastern Buncombe County (see distribution map above). Distribution Reference: Pyron and Beamer (2022)County Map: Clicking on a county returns the records for the species in that county.
GBIF Global Distribution
Key Habitat Requirements
Habitat: Local populations of this and other members of the Black-bellied Salamander complex are strongly associated with perennial mountain streams and stream-seepage complexes. The known habitats range from headwater tributaries and seepages to large trout streams with rapidly flowing water. Members of the complex often found in swifter current than most other Desmognathus species, and can reach high densities in small, unsilted streams with numerous rocks, cobble, and coarse woody debris (Petranka 1998). Populations are most commonly found at elevations from 490-1676 m (Hairston 1949, Organ 1961a), but may occur at lower elevations if local conditions are suitable. The adults and juveniles are typically found directly in or near flowing water, but large adults sometimes forage on the forest floor -- particularly at high-elevation sites. They are only rarely found more than 10 meters or so from a stream bank, and usually much closer when foraging on land.
Life History and Autecology
Breeding and Courtship: Verrell (2006) described the courtship behavior of D. amphileucus from Macon County that were collected in May-June. The orientation phase involves the male slowly and repeatedly approaching the female and following her if she moves away. Once she stops moving, the male stimulates her by nudging her and rubbing her body and head (cheek-to-cheek) for several minutes or more. He eventually slides his body forward under the female’s chin while undulating his tail laterally. When her chin is resting on the base of his tail, the male curves his head and body backwards in a C-shaped posture towards the female’s neck or dorsum. With his tail still undulating, he delivers a forceful single snap of his body that lacerates the skin and delivers pheromones to the female. The force is often sufficient to sometimes flip the male several centimeters away from his partner. This may be repeated several time before the two engage in a tail-straddle walk in which the male slides his head and body underneath the female’s head. She then straddles the distal part of the male’s undulating tail. The pair moves forward, and the male eventually deposits a spermatophore on the substrate. He then swings his tail laterally and moves forward to lead the female to the spermatophore. She places the sperm cap into her cloaca, and the pair then separates. Reproductive Mode: Females of the Black-bellied Salamander complex typically attach their eggs singly to the undersides of rocks, stones or other support structures in tight clusters, and often in fast-flowing water that facilitates aeration. Nests have also occasionally been found above the water line in or near the streambed. The eggs can appear as a closely packed monolayer when attached to flat surfaces or as a bilayer when attached to concave or irregular surfaces (Petranka 1998). Each egg is suspended by a short elastic pedicel that is about 3 mm long and 1.5 mm in diameter. Two clutches from the Great Smoky Mountains (D. gvnigeusgwotli ) and Nantahala Mountains (D. amphileucus ) in North Carolina each contained several subclusters of 2-8 eggs that were attached to a common stalk (Smith et al. 1996a). D. kanawha or D. mavrokoilius in Virginia on 22 June in early to middle stages of development (Organ 1961a), 10 nests of a mixture of D. amphileucus and D. mavrokoilius in western North Carolina between 13 June and 6 August in moderate to advanced stages of development (Pope 1924), and several clutches at or near hatching in early August that were either D. amphileucus or D. mavrokoilius (Orr and Maple 1978). Austin and Camp (1992) found eggs of D. amphileucus in May and hatchlings that were 16 mm SVL in July in a Georgia population. In most instances, females were found guarding their egg masses. Nests discovered by Pope (1924) contained from 26-38 eggs and averaged 32 eggs, while those observed by Organ (1961a) varied from 21-43 and averaged 31. Collazo and Marks (1994) reported an average of 54 eggs (52-56) for five females that oviposited in the both the field and laboratory (one after hormonal injection). As seen in other Desmognathus species, clutch size of the members of this complex is positively correlated with female SVL (Tilley 1968).Aquatic Life History: Hatchlings of the Black-bellied Salamander complex have conspicuous yolk masses and live on their yolk reserves for 1-2 months after hatching (Petranka 1998). The larvae mostly feed on small invertebrates and incorporate larger prey into the diet as they grow. Davic (1991) found that most (82%) of the diet of larval D. amphileucus consisted of aquatic prey, including mayflies, stoneflies, craneflies, chironomids, nematodes, collembolans, crayfishes, and salamander larvae. Large larvae of D. amphileucus will also eat the larvae of the Blue Ridge Two-lined Salamander in experimental settings (Beachy, 1994). Small larvae are often common in perennial seepages and under cobble and small to medium-sized rocks in first and second-order streams. Davic and Orr (1987) estimated the total densities of larval, juvenile, and adult D. amphileucus in streams in western North Carolina to be 5.6-11.7 individuals/m2 of stream bed. (Bruce (1985a) found that small larvae of D. amphileucus often drift or are displaced downstream during flood events, and older larvae tend to prevail in fast-flowing sections of higher-order streams. D. kanawha or D. mavrokoilius since his study site was at the boundary between the ranges of these two species. Bruce (1985a) estimated the larval period of D. amphileucus in one western North Carolina population to be approximately 3 years, but local populations of D. amphileucus vary significantly in length of the larval period and size at metamorphosis (Bruce 1988a, 1989). Individuals usually metamorphose after 2-4 years of growth, but most appear to have a 3-year or 4-year larval period. At a low elevation site in Georgia, the larval period of D. amphileucus lasts nearly 3 years and larvae transform in May and June when they average 40-43 mm SVL (Austin and Camp 1992, Petranka 1998). Terrestrial Life History: After metamorphosing the juveniles and adults of members of the Black-bellied Salamander complex tend to remain in stream beds, seepages, and other aquatic habitats. The juveniles often abound in seepages and headwater streams with moderate water flow and numerous small stones that serve as cover, while the adults are more likely to be found in streams under large rocks or logs, or in burrows in the streambanks (Petranka 1998).D. gvnigeusgwotli that they observed at night along a stream in the Great Smoky Mountains were > 5 m from streams and several animals were > 10 m from the stream's edge. Camp and Lee (1996) studied a population of D. amphileucus in the upper Piedmont of Georgia and found that individuals < 70 mm SVL were mostly found in the stream. Larger animals frequently resided in rock crevices or burrows in the stream bank where they ambushed passing prey. Individuals had small home ranges and appeared to be territorial. D. kanawha or D. mavrokoilius since the study was conducted in the zone of contact between these species. Bruce (1988a, 1989) estimated the minimum age at first reproduction of D. amphileucus in western North Carolina populations to be 6 years for males and 7 years for females. Age-specific survivorship is relatively constant for metamorphosed males, but increases after females reach sexual maturity (Organ 1961a). D. amphileucus feed primarily on aerial prey. Only 36% of prey were aquatic organisms, while the adults fed mostly on terrestrial species. Terrestrial organisms eaten by juveniles and adults include hymenopterans, lepidopterans, beetles, centipedes, and spiders. Hairston (1949) found a variety of aquatic and terrestrial insects in specimens from North Carolina including stoneflies, flies, ants, collembolans, and bugs. These appeared to specimens of D. mavrokoilius . Black-bellied salamanders in northern Georgia consumed stoneflies, mayflies, caddisflies, true flies, beetles, hymenopterans, and other salamanders (Martof and Scott 1957). Eurycea , Desmognathus , and Plethodon that inhabit stream and streamside communities (Camp 1997). Bishop (1924) found four salamanders (including a Plethodon ) in five specimens that he examined, while Formanowicz and Brodie (1993) found that relatively small D. ocoee were most vulnerability to attack or injury by D. amphileucus . Owen McConnell photographed an adult D. amphileucus feeding on a Plethodon teyahalee in Graham County.
General Ecology
Community Ecology: Hairston (1986) experimentally lowered densities of D. ocoee and D. monticola on study plots in the Nantahala Mountains to determine if competition or predation is the major organizing force in a Desmognathus community comprised mostly of D. ocoee , D. monticola , and D. amphileucus . When D. ocoee was removed from plots, the abundance of D. monticola and D. amphileucus decreased relative to control plots. Removal of D. monticola results in an increase in D. ocoee . Desmognathus ocoee also shifted its distribution towards streams when larger congeners decreased in numbers. Hairston (1986) concluded that predation, rather than competition, is the major force that has driven the evolution of body size and habitat preference in the genus.
Adverse Environmental Impacts
Status in North Carolina
NHP State Rank: [S5]Global Rank: GNRStatus Comments: This southern Appalachian endemic occurs primarily in the Blue Ridge Mountains in southwestern North Carolina, extreme northwestern South Carolina, extreme southeastern Tennessee, and northeastern Georgia. It is a common species throughout its range and reaches its highest local densities in high-quality small streams with protected forest buffers. Many local populations are on federal lands and inhabit streams that are minimally impaired.

 »
»



 »
»


