|
|
|
|
|
|
|
|
Photo Gallery for Hyla chrysoscelis - Cope's Gray Treefrog
| 43 photos are available. Only the most recent 30 are shown.
|
 | Recorded by: K. Sanford
Bertie Co. |  | Recorded by: Simpson Eason
Durham Co. |
 | Recorded by: Trey Jeffers
Guilford Co. |  | Recorded by: B. Bockhahn
Rockingham Co. |
 | Recorded by: Andrew W. Jones
Polk Co. |  | Recorded by: Andrew W. Jones
Polk Co. |
 | Recorded by: B. Bockhahn
Sampson Co. |  | Recorded by: Steve Hall, Carol Tingley, Tom Howard, David George, Pat Coin, Jeff Niznik,
Chatham Co. |
 | Recorded by: Stephen Hall
Durham Co. |  | Recorded by: K. Bischof
Transylvania Co. |
 | Recorded by: Andrew W. Jones
Yadkin Co. |  | Recorded by: Andrew W. Jones
Yadkin Co. |
 | Recorded by: David George
Chatham Co. |  | Recorded by: Caleb Garner
Wake Co. |
 | Recorded by: Caleb Garner
Wake Co. |  | Recorded by: David George
Orange Co. |
 | Recorded by: David George
Chatham Co. |  | Recorded by: David George
Chatham Co. |
 | Recorded by: Travis McLain
Cabarrus Co. |  | Recorded by: K. Williams
Surry Co. |
 | Recorded by: Chuck Smith
Davidson Co. |  | Recorded by: Chuck Smith
Davidson Co. |
 | Recorded by: John Petranka
Orange Co. | 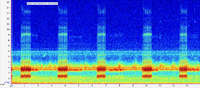 | Recorded by: Jim Petranka and Becky Elkin
Beaufort Co.
Comment: Spectrogram of a single male calling from a roadside ditch. |
 | Recorded by: Steve Hall
Durham Co. | 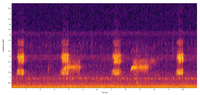 | Recorded by: Steve Hall and Dee Stuckey
Orange Co. |
 | Recorded by: Dee Stuckey and Steve Hall
Orange Co. |  | Recorded by: Mark Basinger
Wilson Co. |
 | Recorded by: Mark Basinger
Wilson Co. |  | Recorded by: Rob Van Epps
Mecklenburg Co. |
|

 »
»



 »
»

