Taxonomy
Class: AmphibiaOrder: CaudataFamily: PlethodontidaeSubfamily: Plethodontinae
Taxonomic Comments: Members of the genus Desmognathus are commonly known as dusky salamanders because of their overall dark brown or dusky ground color. Like many plethodontid salamanders, they have proven to be a taxonomically challenging group that contains several species complexes. Kozak et al. (2005) documented 35 major lineages in the eastern US, even though only 22 species were formally recognized by taxonomists in 2021. This suggests that there are numerous cryptic species that remain to be described. A more recent comprehensive molecular survey of populations in the eastern US by Beamer and Lamb (2020) indicate that at least 45 major lineages or clades are present. Pyron et al. (2020) built upon this data set and incorporated information on nuclear DNA. They suggest that as many as 49 lineages might exists that qualify as candidate species. Although some of the currently recognized Desmognathus species are well supported by both data sets, many are not and likely represent species complexes. Discordance between mtDNA and nuclear DNA analyses are problematic and likely reflect widespread gene flow and reticulate evolution among many Desmognathus lineages. D. carolinensis , D. imitator , D. ocoee , and D. orestes ) have been treated as members of an informal cryptic species complex known as the D. ochrophaeus complex (Tilley and Mahoney 1996). The members of this group are commonly referred to as mountain dusky salamanders, and were previously treated as a single species (D. ochrophaeus , sensu lato) before being split. The most recent molecular study based on mtDNA sequence data (Beamer and Lamb 2020), as well as previous studies (see Beamer and Lamb 2020), indicate that there are many additional clades within the D. ochrophaeus complex that may represent undescribed cryptic species. In addition, certain forms appear to be more genetically similar to D. fuscus than to other members of the D. ochrophaeus complex. Some of the highlights of this study are that 1) Populations currently referred to as D. carolinensis do not appear to constitute a genetically distinct lineage and instead cluster with 39 populations of D. fuscus , 2) Populations currently referred to as D. ocoee contain several clades that may reflect additional cryptic species; in some cases these clades contain mixtures of populations that are currently referred to as D. ocoee , D. conanti and D. apalachicolae , 3) Populations currently referred to as D. orestes contain three clades, one of which is sister to a clade containing D. ochrophaeus and other clades of D. orestes , and 4) Populations currently referred to as D. imitator constitute a well-defined clade that is not closely related to other members of the D. ochrophaeus complex and should not be treated as a member of that complex. Desmognathus species). As additional studies emerge, the taxonomic status of D. carolinensis , D. ocoee , and D. orestes as currently recognized will likely change. Here, we continue to recognize these forms until additional studies are completed and formal taxonomic changes are made. Species Comments:
Identification
Description: Desmognathus carolinensis is indistinguishable in terms of external coloration, patterning and morphology from members of the D. ocoee complex and D. orestes . These species have mutually exclusive ranges and are best identified based on the geographic locality of the collection site. All are medium-sized Desmognathus with tails that are unkeeled, slightly longer than the body, and typically rounded or oval-shaped in cross-section on the proximal half. The distal half can vary from being rounded to more laterally compressed towards the tip depending on the age and condition of an individual. The toe tips lack cornifications as seen in some of the more aquatic Desmognathus species. The adults vary from about 7-11 cm TL and average slightly longer than the females. Technical Reference: Tilley and Mahoney (1996) Online Photos: Google iNaturalist Observation Methods: Individuals are easy to collect by searching beneath cover objects along stream margins or in the adjoining woods. They are most easily observed at night when they actively forage and seek mates on the forest floor. AmphibiaWeb Account
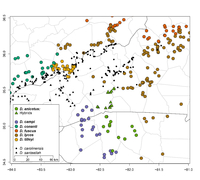
Distribution in North Carolina
Distribution Comments: The Carolina Mountain Dusky Salamander is confined to mountainous, forested habitats in the southwestern Blue Ridge Physiographic Province, including the Blue Ridge, Black, Bald, and Unaka Mountains. Its range extends from a region between Linville Falls and McKinney Gap on the Blue Ridge Divide (Burke and Mitchell counties, North Carolina), and the valley of the Doe River on the North Carolina-Tennessee border, southwestward to the valley of the Pigeon River in Haywood and Buncombe counties (see distribution map above). Distribution Reference: Tilley and Mahoney (1996) County Map: Clicking on a county returns the records for the species in that county.
GBIF Global Distribution
Key Habitat Requirements
Habitat: This and other members of the D. ochrophaeus complex that occur in western North Carolina are associated with montane hardwoods with cool, clear-flowing headwater streams and seepages (Petranka 1998). They also inhabit wet rock faces. Brooding females and overwintering juveniles and adults congregate in aquatic habitats, but non-brooding individuals move away from aquatic habitats and live on the forest floor during the warmer months of the year. At sites where mesic conditions prevail, they can be found far from water. At lower elevations, individuals tend to remain in the immediate vicinity of seeps and streams (Beane et al. 2010, Petranka 1998). Biotic Relationships: Desmognathus carolinensis is palatable to predators and is likely preyed upon by woodland birds, small snakes, and large salamanders that live in streamside habitats in mesic forests. The Spring Salamander (Gyrinophilus porphyriticus ), Black-bellied Salamander (D. quadramaculatus ), and Seal Salamander (D. monticola ) feed on other members of the D. ochrophaeus complex (Bruce 1979, Coker 1931, Formanowicz and Brodie 1993, Hairston 1986, Whiteman and Wissinger 1991) and undoubtedly also feed on D. carolinensis .
Life History and Autecology
Breeding and Courtship: Mating of this an other members of the Mountain Dusky Salamander complex in western North Carolina occurs during the spring, late-summer, and autumn, but seasonal patterns for D. carolinensis are poorly documented. Females oviposit annually and courtship involves a stereotypic tail straddle walk that is typical of plethodontids. Details of courtship behavior have not been published, but courtship is presumably very similar to that described under the account for D. orestes . Females appear to mate several times per year and are capable of long-term sperm storage in their cloacas (Petranka 1998). Reproductive Mode: Females deposit their eggs in small grape-like clusters in hollowed depressions that are constructed beneath cover. They can be found beneath rocks, decaying logs, leaf litter, and in moss mats and debris within or in the immediate vicinity of springs, seeps, and small streams (Martof and Rose 1963, Tilley 1973b). Each egg has a short gelatinous stalk and is attached to a common attachment point to form a cluster. The mature ova are typically no more than 2.9-3.2 mm in diameter (Petranka 1998). The females often gather in large congregations in seepages or other wet microhabitats and construct their nests within a few centimeters or decimeters of each other (Tilley 1973b). The clutch sizes of eight populations studied by Tilley (1973a) averaged from 13-26 eggs, while two populations studied by Martof and Rose (1963) averaged 12.5 and 28 eggs per clutch. Clutch size is positively correlated with female body size and increases with elevation (Tilley 1973a). Aquatic Life History: The larvae live in seepages, in slow sections of headwater streams, and on wet rockfaces, often in very shallow water. The larval period typically lasts 2-8 months depending on the time of oviposition and elevation. Hatchlings from eggs laid in the late winter or early spring transform in 2-3 months, while those from eggs laid in the summer may overwinter and transform 4-8 months later (Tilley 1973b). Published dietary studies of larvae are not available, but the larvae presumably feed on small invertebrates such as midge larvae. Terrestrial Life History: The juveniles and adults congregate in moist underground retreats such as seepages and headwater streams and streambanks during the winter months. With the onset of warmer seasonal weather most disperse to the surrounding forest except for nesting females that remain in close proximity to running water (Tilley 1973b). Individuals may move 40 m or more into the adjoining forests and can reach high densities in streamside habitats (Petranka and Smith 2005). Petranka and Murray (2001), for example, estimated densities to be 1.07 salamanders/m2 on the forest floor in a mesic cove forest in western North Carolina. D. carolinensis sometimes feed on their own eggs and hatchlings. Although they will preferentially eat dead eggs introduced into their clutches, they occasionally eat healthy eggs from their own broods immediately after consuming a dead egg (Tilley 1972). Hatchlings are sometimes cannibalized, and in some instances females of this and other members of the D. ochrophaeus complex appear to eat their own young (Petranka 1998).
General Ecology
Population Ecology: Local populations are centered around seepages, wet rockfaces, and headwater streams and often consist of thousands of individuals. Connectivity between local and regional populations may be hampered by physiological barriers such as dry ridges, fish-filled lower areas of drainages, or hot, dry valleys. This species typically exhibits genetic isolation by distance and has strongly differentiated regional populations which suggest that gene flow at larger spatial scales is often relatively low.
Adverse Environmental Impacts
Habitat Fragmentation: Forest fragmentation can not only directly eliminate critical habitat for this species, but also generate edge effects that can degrade terrestrial habitat. This and other plethodontid salamanders rely on their moist skin as a respiratory organ to obtain oxygen and are sensitive to physiological stress. Forests that adjoin pastures, agricultural fields, roadways or clearcuts tend to be hotter and drier along their margins relative to interior regions. These edge effects can extend 30 meters or more into a forest and often result in reduced salamander numbers along forest margins (e.g., Crawford and Semlitsch 2007, Demaynadier and Hunter 1998, Semlitsch et al. 2006).
Status in North Carolina
NHP State Rank: S3S4Global Rank: G4Environmental Threats: This and other plethodontid salamanders lack lungs and rely on their moist skin as a respiratory organ to obtain oxygen. Mature and old-growth forests that have high-quality headwater streams and seepages, thick layers of leaf-litter, rich organic soils, abundant surface cover, and heavily shaded canopies prove ideal conditions for this and other woodland salamanders. D. carolinensis and other salamanders that inhabit streamside communities due to the loss of leaf litter, surface cover, and the creation of drier and hotter site conditions. Recovery of populations to precut levels in Appalachian forests often require several to many decades as regenerating forests slowly develop into mature stands (Connette and Semlitsch 2013, Crawford and Semlitsch 2008, Hicks and Pearson 2003, Petranka et al., 1993, 1994). Hicks and Pearson (2003) examined how past land uses have impacted this species in North Carolina. At reforested sites that were previously logged or farmed, populations were still depressed relative to undisturbed sites after about 50 years of recovery from past disturbances. Status Comments: Desmognathus carolinensis is often one of the most common species in the Appalachian Mountains in mesic cove forests and on rich slopes. Many lower-elevation populations have been lost due to the conversion of land to pastures, agricultural fields, and urban development. However, in general this species is abundant within its range and in minimal need of protection. Stewardship: Local populations are best maintained by keeping a wide riparian zone of mature forest along headwater streams and seepages. The juveniles and adults spend much of their time during the summer months on the forest floor and forest buffers of 50 meters or more are needed to provide suitable habitats and minimize edge effects.
Photo Gallery for Desmognathus carolinensis - Carolina Mountain Dusky Salamander 15 photos are shown. Recorded by: B. Bockhahn Recorded by: tom ward Recorded by: tom ward Recorded by: tom ward Recorded by: tward@taroman.com Recorded by: tward@taroman.com Recorded by: tom ward Recorded by: tom ward Recorded by: tom ward Recorded by: tom ward Recorded by: B. Bockhahn, CHRO staff Recorded by: Steve Hall Recorded by: Steve Hall Recorded by: Jim Petranka Recorded by: Jim Petranka

 »
»



 »
»


