Taxonomy
Class: AmphibiaOrder: AnuraFamily: HylidaeSubfamily: HylinaeOther Common Name(s): Eastern Gray Treefrog
Taxonomic Comments: Duellman et al. (2016) elected to split treefrogs in the genus Hyla into two genera. Hyla (sensu stricto) refers to a group of species that are found in Eurasia, while a new genus Dryophytes was resurrected to include all of the North and Central American species and three other species that are found in eastern temperate Asia. Members of these proposed genera cannot be distinguished by any known morphological features, but represent two clades. Whether members of these clades should be placed in separate genera or treated as lesser taxa is arbitrary and dependent on one's taxonomic philosophy. Here, we retain Hyla for North American species to be consistent with current usage by the Society for the Study of Amphibians and Reptiles. Hyla chrysoscelis ) and the Gray Treefrog (H. versicolor ) are two morphologically indistinguishable species that are members of a polyploid complex. Hyla chrysoscelis is a diploid species (2N = 24 chromosomes), while H. versicolor is a tetraploid (4N = 48 chromosomes). The exact origin of H. versicolor has intrigued evolutionary biologists and generated several hypotheses that are supported to varying degrees by molecular and genetic analyses. Holloway et al. (2006) proposed that H. versicolor arose via several speciation events from H. chrysoscelis -like diploid ancestors and two other now extinct lineages of tree frogs. A recent analysis that used a much more comprehensive set of molecular data suggests that H. versicolor most likely formed via a single genome duplication event that involved only H. chrysoscelis and did not involve hybridization with other species (Booker et al. 2021). Since then, there has been significant hybridization between diploids and tetraploids where they co-occur. This has led to the reformation of distinct polyploid lineages following the initial whole genome duplication event. Despite evidence of a complex evolutionary history that involves the repeated re-formation of H. versicolor lineages through time, researchers treat the diploid and tetraploid forms of this complex as only two species. Since they are morphologically identical, the two species can only be differentiated using other evidence such as the chromosome number or voice characteristics. Fortunately, these species are rarely found in sympatry in North Carolina and can usually be identified based on locality. Hyla chrysoscelis occurs statewide, but H. versicolor is only known from a northern tier of counties in the Piedmont near the Virginia border, and from one record from Rowan County in the Piedmont. Species Comments: Hyla versicolor has served as a model organism to study acoustic communications in anurans, with dozens of papers now published on this topic. As noted by Dodd (2013), many of the older publications concerning gray treefrogs are compromised because of the inability to determine if the species that was studied was H. versicolor or H. chrysoscelis . This is a particular problem in areas of sympatry where both species could potentially occur at a study site.
Identification
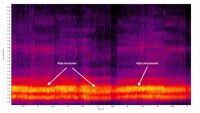
Description: Hyla versicolor and H. chrysoscelis are morphologically indistinguishable and the following description of H. versicolor applies equally to both species. The Gray Treefrog is a small to medium-sized frog with conspicuous toe pads, granular skin, and a lichen-like dorsal pattern. The dorsal patterning of the adults often consists of a grayish ground color that is overlain by darker patches with thin black margins. However, the ground color can vary from ashy white to pale brown or greenish, and individuals can change body color to better match their backgrounds (Beane et al. 2010, Dodd 2013). The juveniles generally tend to be greener than the adults, and often have rather poorly developed dark patches. There is a conspicuous whitish patch underneath the eye that is often lined on the sides with a thin black line. The upper surface of the legs have dark bands and the rear toes are partially webbed. The concealed surfaces of the back legs are mottled with bright orange and blackish pigmentation near the margins and washed with bright orange elsewhere. The belly, throat, and undersides of the front legs are whitish and unmarked, except for males which have dark vocal sacs during the breeding season. Hyla versicolor is a slightly larger species than H. chrysoscelis but there is wide overlap in the size ranges (Dodd 2013). As with H. chrysoscelis , the males average slightly smaller than the females. In Connecticut, the males vary from 36–51 mm SUL (mean = 46 mm) versus 43–60 mm SUL (mean = 50 mm) for females (Klemens 1993). In Ohio, the means for males from several ponds ranged from 43-46 mm SUL versus 49–53 mm SUL for females (Gatz 1981b). Beane et al. (2010) reported a range of 32-62 mm SUL for populations in Virginia and North Carolina. H. chrysoscelis (Altig 1970). The hatchlings vary from 4-7 mm TL and are brownish with black mottling on the tail. There is a pair of preorbital stripes, but they are not distinctly outlined and only become apparent after two weeks (Dodd 2013). As the tadpole grows, the venter becomes more cream colored with a heavy intrusion of gold flecks on the posterior end. The maximum size is around 42 mm TL (Dodd 2013). This species has inducible defenses against predators. Tadpoles in ponds with predatory dragonfly larvae respond by developing relatively large, brightly colored tail fins with blackish spots or heavy mottling along the margins (McCollum and Van Buskirk 1996). So, expect significant variation depending on local site conditions. Hyla versicolor and H. chrysoscelis can only be distinguished in the field by the trill rates of calling males (pulses per second). We recommend making recordings of calling males for later analysis at any site where this species appears to be present. Mitchell and Pague (2011) conducted an extensive survey of populations in Virginia and found that there is no overlap in the trill rates of the two species when recorded in the field (i.e., prior to adjusting for temperature differences). The mean and standard deviation for trill rates for H. chrysoscelis was 45.8 (4.9) versus 24.5 (3.4) for H. versicolor . In essence, any field recording that shows a trill rate of < 30 pulses/sec can be assigned to H. versicolor with very high confidence. Vocalizations: The advertisement call is a very loud slow trill. The trill of this species is lower pitched and occurs at a slower pulse rate (17–35 notes/sec) than that of H. versicolor (34–69 notes per second). The pulse rate varies depending on the ambient temperature and location, but averages between 24 and 29 pulses/sec at 24°C (Dodd 2013). Males that were recorded in the field in Virginia averaged 24.5 pulses/sec at an average air temperature of 22.5°C (Mitchell and Pague 2011). Each trill last less than a second, and sequential trills are typically issued every 2-7 seconds. Technical Reference: Dodd (2013)Online Photos: Google iNaturalist Observation Methods: Individuals are most easily observed around the breeding sites at night during rainy weather. They are also commonly seen crossing roads on rainy nights as they move to and from the breeding sites.
Download Video:
"MP4"
AmphibiaWeb Account
Distribution in North Carolina
Distribution Comments: Hyla versicolor tends to have a more northern distribution than H. chrysoscelis , although some populations occur as far south as southeastern Texas and there are broad areas of sympatry (Dodd 2013). The range extends from western New Brunswick westward across southern Canada to western Manitoba and eastern Saskatchewan. From there is extends southward to central Virginia and north-central North Carolina in the east, and to northeastern West Virginia, south-central Ohio, south-central Indiana, and much of Illinois and Missouri to the west. A southern band continues from Missouri through southeastern Kansas, eastern Oklahoma, and northwestern Arkansas into eastern Texas and southwestern Louisiana. Apparent disjuncts are present in northern Kentucky and western Tennessee. As of 2022, populations have only been located in North Carolina in four counties along the Virginia border and at a site in Rowan County. Distribution Reference: Beane et al. (2010), Dodd (2013)County Map: Clicking on a county returns the records for the species in that county.
GBIF Global Distribution
Key Habitat Requirements
Habitat: This highly arboreal species is most commonly associated with deciduous hardwood forests where it blends in well with tree limbs in the forest canopy. Its habit preferences overlap strongly with that of H. chrysoscelis and the two species often occur syntopically. Adult H. versicolor frequent mesic and bottomland hardwood forests, mixed hardwood-conifer forests, as well as boreal forests in Canada. Breeding sites are either embedded within the forest proper or occur in more open habitats that typically are < 50 m or so from a forest tract. H. chrysoscelis . The adults generally prefer fish-free seasonal or semi-permanent wetlands for breeding, but will use permanent ponds with predatory fish to a lesser extent (Dodd 2013). Commonly used habitats include vernal ponds in forests, marshes, flooded fields, floodplain overflow pools, red maple and shrub swamps, beaver ponds, roadside ditches, quarry ponds, flooded sand pits, farm ponds, and the shallow sections of lakes. Wright (1914) noted that males in New York frequently call from ponds with floating aquatics such as lily pads that served as resting or calling perches. Hocking and Semlitsch (2007) found that females preferred artificial pools set up in open habitats rather than in shaded forested sites. They most frequently used pools that were within 10 m of the forest edge. H. versicolor from the Coastal Plain of Virginia and North Carolina (Beane et al. 2010, Mitchell and Pague 2011) suggests that this species may be less tolerant of acidic, blackwater breeding habitats than H. chrysoscelis . However, the eggs and larvae are fairly acid tolerant, so the reason for their seeming absence from these regions is poorly understood. Environmental and Physiological Tolerances: This species has a more northerly range than Cope's Gray Treefrog, which may reflect a greater degree of cold-tolerance. Hyla versicolor does not occur in most of the Atlantic Coastal Plain or the Gulf Coastal Plain which suggests that is may be less tolerant of acidic waters for breeding. However, the eggs and larvae appear to be acid tolerant, with the a critical pH level of 3.5-4.3 for larval and 3.8 for embryos (Dodd 2013, Gosner and Black 1957a, Grant and Licht 1993). Pehek (1995) found that exposure to a low pH (3.9) has no effect on survival, body mass, growth rates, length of the larval period for specimens from New Jersey.Biotic Relationships: As with H. chrysoscelis , the larvae are palatable to fishes and a variety of other aquatic predators such as insect larvae, mole salamander larvae and newts (Adams et al. 2011, Gunzburger and Travis 2005, Van Buskirk and McCollum 2000, Walters 1975). The tadpoles are frequently killed and eaten by aquatic invertebrates and have evolved inducible defenses that reduce attack success. When confined with dragonfly larvae, the developing tadpoles develop relatively large, brightly colored tail fins with dark spots and blotches that are concentrated along the margins. These entice predators to strike the tail region rather than the more vulnerable head and body. Ambystoma larvae (Van Buskirk and McCollum 2000). Schoeppner and Relyea (2005) examined how tadpoles altered their defenses when 10 different prey of a dragonfly predator were either crushed by hand to release alarm chemicals or consumed by the predator. They found that crushed and consumed prey both caused the tadpoles to decrease their activity and hide more, but only the consumed prey consistently induced deep tails and shorter bodies. Argiope sp.) occasionally eat the small metamorphs (Groves and Groves 1978), and the juveniles and adults are undoubtedly eaten by birds, aquatic snakes, bullfrogs, skunks, raccoons, and other mammals. The juveniles and adults blend in very well with their substrates and presumably reduce predation risk by remaining immobile in a crouched position. The sudden display of the bright coloration on the inside of the thighs as the frog jumps may serve to startle a predator long enough for the frog to escape (Dodd 2013). The adults have noxious skin secretions that can irritate the eyes and mucous membranes of human handlers. H. chrysoscelis , the breeding adults can reduce the adverse impacts of predators and competitors on their offspring by selecting oviposition sites that are favorable. Fish will readily consume the larvae (Adams et al. 2011, Kurzava and Morin 1998, Smith et al. 1999) and the adults are less likely to use ponds with predatory fishes (Babbitt et al. 2003, Baber et al. 2004, Dodd 2013). Smith (2021) found that females are less likely to oviposit in artificial pools with the Western Mosquitofish (Gambusia affinis ) and Shulse et al. (2013) found that mosquitofish greatly reduced the abundance of Gray Treefrog larvae in constructed ponds. At sites where clusters of ponds are present, pond use can shift between years (Brodman 2009, Skelly et al. 2003). This suggests that adults monitor risks to their offspring prior to breeding and frequently shift to alternate breeding sites that maximize the growth and survival of their future offspring.
Life History and Autecology
Breeding and Courtship: The males typically begin calling after the spring leaf-out and may continue through August, although most breeding occurs during the early part of the breeding season. Calling often does not begin until late April or May -- or even later at northern latitudes -- but can begin in March in Texas (Dodd 2013). The males descend from trees and make their way to the breeding sites where they set up calling stations either at ground-level or in low vegetation. They often call from shrubby vegetation or small trees in or near the pond, or from emergent or floating vegetation (Bertram et al. 1996). Dodd (2013) noted that chorusing in many local populations tends to be of short duration. This likely reflects the short growing season at many northern locales and the need to complete the larval stage prior to the onset of cold weather. Examples include 12–23 days in Maine (Sullivan and Hinshaw, 1992) and 13–37 days in Ontario (Bertram and Berrill 1997). A population studied by Fellers (1979b) in Maryland bred for around 7 weeks. Reproductive Mode: Egg laying has yet to be described in detail, but is presumed to be very similar to that of H. chrysoscelis . The female deposits several rafts of perhaps 20-100 eggs in a monolayer on the water surface, and will often swim away to a nearby location in the pond after ovipositing a group of eggs. Dodd (2013) noted that the entire complement of eggs can be laid in 5-10 minutes, but can take up to an hour in some instances. Aquatic Life History: Very little is known about the larval stage except for the well-documented phenomenon of inducible defenses. The larval period in natural populations is reported to last around 40–60 days (Gosner and Black 1957b), and recent metamorphs vary from 13.6–20 mm SUL (mean 16 mm) in New York (Wright 1914). Metamorphs examined by Munz (1920) in New York varied from 19-21 mm SUL.Terrestrial Life History: Roble (1979) found that most metamorphs at a Wisconsin site dispersed away from breeding ponds within a week after metamorphosing, then remained within 125 m of their natal ponds. Most were found < 1 meter above ground on the top surfaces of the leaves of sedges, false nettle, reed canary grass, young swamp oak saplings and other low-growing vegetation. Frogs were commonly found from late July through late September, but appeared to move to overwintering retreats by early October. Individuals in this study did not appear to be arboreal during their first year of life.
General Ecology
Population Ecology: Local breeding populations often consist of only a few hundred adults or less (Dodd 2013). Where clusters of local ponds occur locally substantial gene flow likely occurs due to dispersal of juveniles and adults between ponds. In Missouri, Johnson (2005) found that there was significant variation in population genetic structuring and that populations > 30 km apart were identifiable. Under 3 km, however, significant structuring was usually not evident. Populations appeared to be structured as classic metapopulations at regional distances, but more as “patchy” metapopulations with substantial interpond movement when clusters of local ponds were in close proximity. Factors that regulate local populations are poorly resolved but may involve both competition among territorial males for mates and density-dependent growth and survivorship during the larval stage. Community Ecology: The larvae usually share breeding sites with other anurans, but little data are available on competitive interactions. Fish and aquatic invertebrates are important predators on the larvae and mortality is minimized through a combination of adult choice of breeding sites, altered behavior, and inducible defenses (see biotic interactions above).
Adverse Environmental Impacts
Habitat Loss: Many local populations have undoubtedly been lost historically due to deforestation and urbanization. Roads present hazards to adults that are moving to and from breeding sites, and run-off from salted roads in northern locales can be toxic to the embryos and larvae of these and other pond-breeding amphibians if salt concentrations are high. Habitat Fragmentation: Gray Treefrogs appear to tolerate habitat fragmentation well so long as forested areas remain that are large enough to support adult populations. Artificial aquatic habitats that are found in disturbed and urbanized landscapes such as flooded ditches, flooded fields and farm ponds with emergent vegetation are often used as breeding sites and have compensated for the loss of natural wetlands.
Status in North Carolina
NHP State Rank: S2Global Rank: G5Status in North Carolina: SCStatus Comments: Regional populations of this species appear to be stable, with no evidence of decline at a landscape scale (Dodd 2013). The status of populations in North Carolina is largely unknown. Populations here are at the southern limit of the range for areas east of the Mississippi River and are in need of additional study and monitoring. Stewardship: Local populations are best maintained by having tracts of deciduous hardwoods next to fish-free breeding sites. Cluster of breeding ponds that are within a few hundred meters of each other allow adults the opportunity to shift to alternate breeding sites in response to fish invasions or other biotic stressors.

 »
»